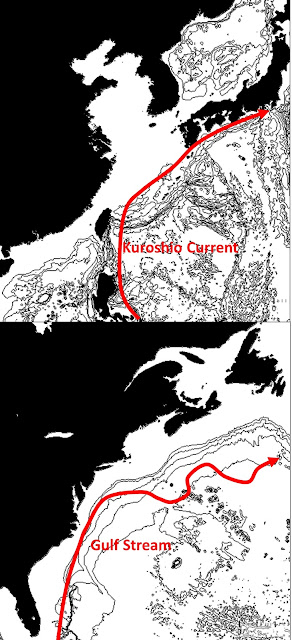
Anti-hibernation

"Humans were never meant to hibernate" - message on a T-shirt I waddled across the snow-covered dock, laden with gear. I was wearing my dry suit and had a SCUBA tank on my back. A regulator and two waterproof lights dangled over my shoulders. On my hips, I carried an extra 10 lb of lead, plus 2 lb on each ankle. I already had my mask and gloves on, but I was carrying my fins. Slowly, I shuffled my feet through the snow, keeping my balance on the wintery pier. The cold air felt good in my lungs. Carl had told me to get in the water as quickly as possible so my regulator didn't freeze up again - we had climbed out to fix it once already. As I approached the edge of the instrument well, I lifted one leg over the wooden barrier, then the other. I leaned on a storage bin to slide on my fins. I shuffled to the edge, put the regulator in my mouth, and... SPLASH! The 41° F water surrounded me. I could feel the cold, salty sting on my neck and my lips, the only p...